Which of the Following Correctly Describes the Energy Balance Equation
0328 mol of ZnS reacts. 158 g of O2 are required to react completely with the ZnS.

Chapter 3a The First Law Closed Systems Energy Updated 1 17 11
The sum of the subscripts on the left of the equation must equal the sum of the subscripts on the right.

. A chemical equation need not be balanced. Kinetic energy is zero. The next energy balance equation is for the turbine of the ORC unit and it is also the same for all systems.
Check all that apply. In a chemical equation reactants and products are represented by symbols or molecular formulas The physical states of. This is a process in which living organisms combine food glucose with oxygen into energy while producing carbon dioxide and water as waste products.
At A only potential energy exists. Since organisms cant use the energy from food directly cellular respiration is necessary to convert the energy. Glucose oxygen carbon dioxide water energy The equation is formulated by.
23592U 10n 9236Kr 14256Ba 210n energy For this reaction the sum of the masses of the products is slightly less than the sum of the masses of the reactants. CO2 C 2CO. Place a coefficient of 2 in front of the O2.
Another possible reaction of U-235 is represented by the incomplete balanced equation below. CO2 C2 CO B. Which of the following correctly describes the energy balance equation aenergy consumed minus energy expended benergy expended minus energy consumed cenergy expended plus energy consumed denergy consumed equals energy expended.
This reaction absorbs 288 kJ of energy. Thus to balance equation multiply CrCl3 with 2 which result in equation as follows 12 K 2 62 Cr 2-27 O 7 -1 H-1 CL HCL 2 3 Cr-13 Cl 3 0 Cl 2 -1 K-1 Cl 12 H 2-2 O. CO2 C C2O.
At C both potential and kinetic energy exist. The above equation predicts that there should be 6 HCl atoms at LHS which undergo change in oxidation state by losing six electrons and obtain an oxidation state of zero in 0Cl2. Log in for more information.
The mass and energy balance equations of the heat exchanger for air heating are given as. CH4 2O2 CO2 2H2O. Write a balanced molecular equation describing each of the following chemical reactions.
Expert answered Jay901 Points 8133. The measure of the number of atoms in one element that will combine with an atom of another element is ValenceUser. Which of the following correctly describes the energy balance equation.
Each side has 1 C atom 4 H atoms and 4 O atoms. 14mair in mair out. 320 g of ZnS reacts according to the balanced equation 2ZnS s 302 g 2ZnO s 2SO2 g.
Which of the following statements correctly describe a chemical equation. Energy in Energy out Change in Body Stores. CO2 C 2CO C.
We now have 4 O atoms on both sides. At B only kinetic energy exists. CO2 C CO.
Select all that apply. 2CO2 C CO D. There are 2 O atoms on the left side and 4 on the right.
So Potential energy at A is equal to potential energy at C and kinetic energy. The equation is now balanced. C_6H_12O_6 O_2 CO_2 H_2O energy The balanced equation is C_6H_12O_6 6O_2 6CO_2 6H_2O energy The equation expressed in words would be.
The overall unbalanced chemical equation for cellular respiration is. In its exceedingly simplest form the energy balance equation is this. The correctly balanced form of this equation is.
16 m7 h 7 mair h air in m8 h 8 mair h air out. Select all that apply. Energy consumed equals energy expended.
This is essentially just a restatement of basic thermodynamics since energy cant be created or destroyed it all has to be accounted for in some form or fashion. B Gaseous butane C 4 H 10 reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. Which of the following options correctly describe how to balance a nuclear equation.
A chemical reaction in which two or more substances undergo a chemical union to form a more. ΔH -879 kJ which of the following options correctly reflect the information given. A chemical reaction in which two or more substances undergo a chemical union to form a more complex substance is a _ reaction Weegy.
A Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas. The equation C6H12O6 6O2 6CO2 H2O energy depicts the process of cellular respiration. Potential energy is zero.
Energy consumed equals energy expended the label of ready-to-eat cereal box indicates that 1 cup contains 32 grams of cars 4 grams of protein 2 grams of fat how many kcals are there in tow cups of cereal.

Ionization Energy Basic Introduction Youtube

Chemistry Reactions Combination Reaction When Two Or More Reactions Combine To Form Chemical Reactions Chemical Reactions

Chapter 3a The First Law Closed Systems Energy Updated 1 17 11

Work And Energy Physics Mechanics Energy Work Physics

A Synthesis Reaction Is A Type Of Reaction In Which Multiple Reactants Combine To Form A Single Product Synthesis React Chemical Reactions Chemistry Synthesis

Speed Velocity And Acceleration Physical Science Lessons Writing Linear Equations Physical Science Middle School
What Is Steady Flow Energy Equation Quora
What Is Steady Flow Energy Equation Quora

Law Of Conservation Of Energy Video Khan Academy
How Much Energy Is Released In Atp Hydrolysis
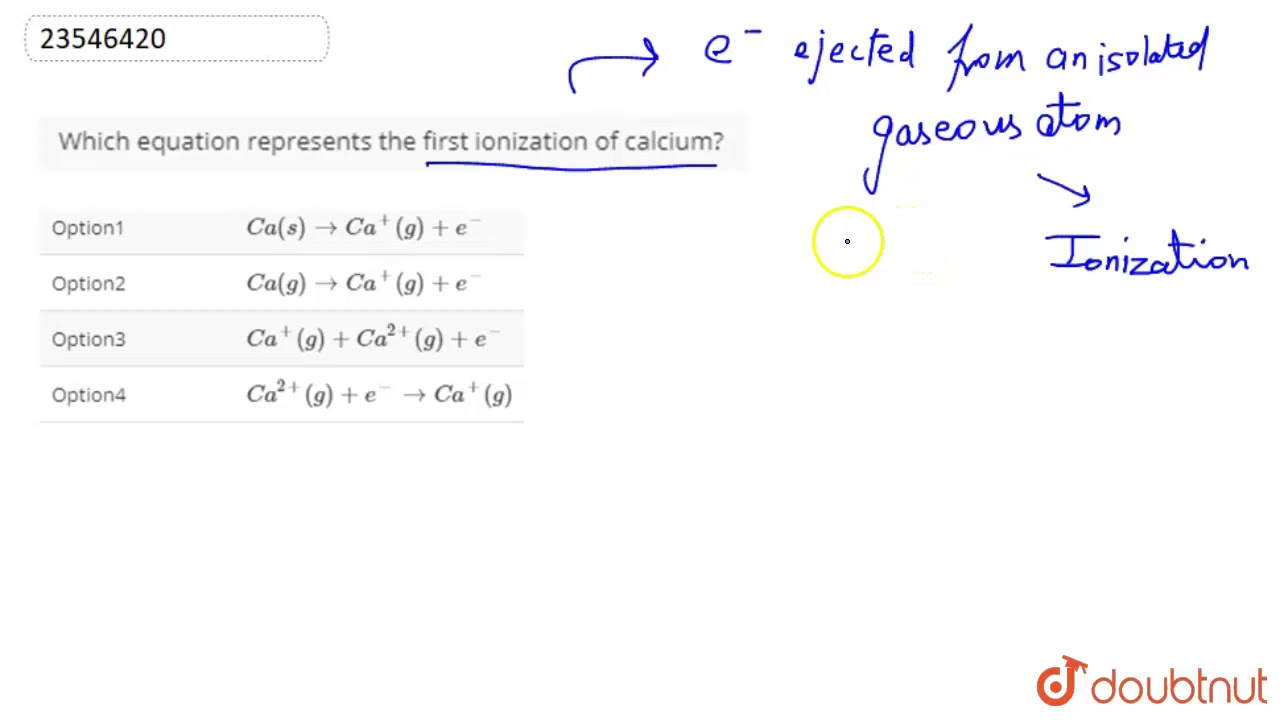
Which Equation Represents The First Ionization Of Calcium Youtube

Steady Flow Energy Equation An Overview Sciencedirect Topics

Chapter 2 The First Law Of Thermodynamics For Closed Systems Thermodynamics

Arrhenius Equation Activation Energy And Rate Constant K Explained Youtube

Conservation Of Energy In Projectile Motion Examples Analysis Video Lesson Transcript Study Com

Using The Conservation Of Energy Theorem To Find An Initial Velocity Physics Study Com

Chapter 3a The First Law Closed Systems Energy Updated 1 17 11
Which Pressure Does The P In Bernoulli S Equation Reference Quora


Comments
Post a Comment